Title
INSIGHT (2)
The Directors, Wayne and Florence Hillman established Insight Medical Products Limited in May 2000.
From our base in Tetbury, Gloucestershire, Insight has become a highly respected supplier of good value, high quality products to the healthcare market.
We are pleased to work with hospital professionals to design and manufacture innovative products that will be of use to hospital departments world-wide.
At Insight Medical Products we have our own in-house manufacturing facility with a fully accredited cleanroom facility for manufacturing and packing.
Our Quality & Regulatory management systems meet with the requirements of harmonised standard ISO 13485. Our devices manufactured at Insight Medical Products conforms to the requirements of Medical Device Directive 93/42/EEC and UK MDR 2002 under the supervision of SGS. We are currently conforming to the upcoming Medical Device Regulations (EU) 2017/745 requirements and will be obtaining certification before the required date.
We are in progress for certification of ISO 14001 under the supervision of BAB.
Medasil Surgical
NEOTECH
Over the past three decades, Neotech has emerged as an essential brand in NICUs around the world. We’ve also widened our footprint to include medical devices for the PICU, children’s oncology, and home health markets. Our groundbreaking products benefit both patients and clinicians as we focus on Making a Difference… In the NICU, PICU and Beyond.
We understand the quality of products used in such a sensitive environment is of the utmost importance to clinicians. We comply to strict FDA and ISO standards.
With such important patients to care for, we wouldn’t leave production to just anyone. Our U.S.-based vendors understand the importance of quality in our products and follow strict quality control protocols. We also carefully monitor quality control through every step of our process, ensuring the highest quality product possible. We will continue to strive to make the highest-quality neonatal and pediatric products on the market.

NGPOD
About NG
At NGPod Global, we are dedicated to providing innovative solutions to the patient safety issues surrounding nasogastric (NG) tube misplacement.
OUR STORY
The young daughter of an inventor fell ill and had to be fed through a Nasogastric (NG) feeding tube. Observing the difficulties and variations in how different nurses confirmed the position of the NG tube before feeding he thought, there must be a better way to do this?
So he set out to improve the care pathway for patients requiring Nasogastric (NG) tubes.
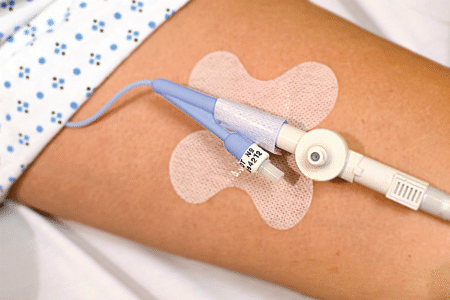
The NGPOD® Device
Using patented fibre-optic sensor technology, the NGPOD® takes the pH test to the distal end of the NG tube inside the patient.
This CE marked device has been designed to be an alternative to conventional feeding tube position confirmation tests – delivering a clear, simple indication that requires minimal clinical interpretation. In particular it increases the percentage of patients who can be pH tested (the recommended first line method in the UK, USA & other countries) as it removes the issue of obtaining aspirate from patients.
The device is intended for use by healthcare professionals for patients who require enteral feeding or medication via nasogastric (NG) feeding tubes in the acute, out-patient or home care environment.
Located in the heart of the Northern Powerhouse, with offices at Sci-Tech Daresbury, Cheshire, and local Manufacturing and R&D Operations.
Best Sellers
Our current Best Sellers
Medutek
Internal Nasal Airway Splint

Internal Nasal Airway Splint
Description


NGPOD
TUBE TESTING
-

NGPOD® Handheld Device
The NGPOD®-01 should be used with the NGPOD® Sensor-01. When used together it will provide the clinician with a simple YES/NO result for indicating placement of a Nasogastric (NG) tube.
As a result, the NGPOD® system removes the need to aspirate gastric contents from the patient, reduces clinical interpretation risk by providing a safe bedside test which gives the user an unambiguous yes or no result. It also reduces the need for unnecessary X-rays to confirm placement and offers patient safety, clinician and cost-efficiency benefits.
-

NGPOD® Disposable Sensor
Using a bespoke fibre-optic, we have developed a patented Fibre-Optic Sensor which we manufacture in the UK.
When used with the NGPOD®-01, the fibre-optic transmits light from the Pod, detecting a response from the environment at the tip of the sensor (which sits inside the distal end of the nasogastric tube once inserted).
The device provides a result in c.22 seconds.
The sensor is single use and must be disposed after each patient use.